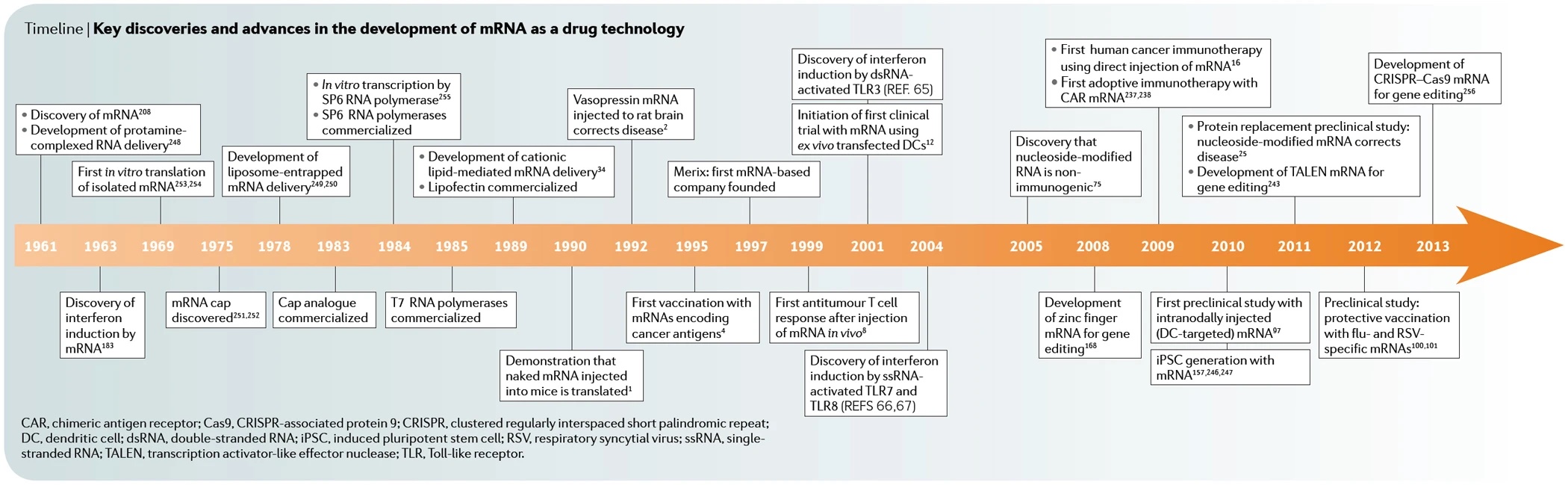
Part 2 of the series on the history of mRNA (vaccines) looked at the head-to-head race in 1961 and what happened in the 1990s. Then, starting in 2000, the first twenty years of the new millennium laid another foundation for today's applications of mRNA technology as a vaccine.
Ingmar Hoerr and his company CureVac
Another chapter in the history of the use of mRNA as a drug or vaccine began in August 2000 with the publication of the scientist Ingmar Hoerr, who used synthetically produced, stabilized and protected mRNA (packaged and unpackaged) to provoke a specific immune response in vivo in mice. At the same time, he founded the world's second mRNA company, Tübingen-based CureVac.
Hoerr had been working on RNA as a potential vaccine since 1996 as part of his doctoral thesis at the German University of Tübingen. Together with Günther Jung, Hans-Georg Rammensee and Reinhard Obst, he filed a European patent application in 1999, which was published in 2001 and granted in 2005. The title: "Transfer of mRNA using polycationic compounds." In this, the applicants already described the use of mRNA as a vaccine.
Further patent applications were filed together with colleagues from the newly founded CureVac, including Florian von der Mülbe and Steve Pascolo. Below is a selection (in parentheses year 20x of application | grant):


Darstellung von der Webseite CureVac
- Method for the preparation of a mRNA tumor antigen library (02|08)
- Stabilized mRNA with increased G/C content and optimized codon usage for gene therapy (02|10),
- Stabilized mRNA with increased G/C content, coding for a viral antigen and their use (02|10) - in Dec 2023 declared as null and void by the German Federal Patent Court
- Transfection of blood cells with mRNA for immunostimulation and gene therapy (04|08)
- RNA-coding antibody (08|16) - it is also possible to administer mRNA that codes for an "own-body-produced" antibody drug
- Composition to treat prostrate cancer (08|17)
- RNA-composition to treat non small cell lung cancer (08|21)
Ingmar Hoerr and other colleagues at CureVac were the first to publish results on the application of mRNA technology to cancer and infectious diseases, starting in the second half of the first decade of the new millennium. In 2005, for example, they published initial results on their attempts to use tumor-specific mRNA or molecules derived from it to combat metastatic skin cancer. In a clinical phase I/II trial initiated in 2003, patients were "vaccinated" with their own tumor mRNA: the immune system was thus "armed" to better recognize and fight the tumor.
Initially, the immune system is still able to eliminate cancer cells. Eventually, however, the point is reached where the tumor takes control. CureVac's mRNA approach as an anti-tumor vaccine was thus the first in the field of cancer immunotherapy.
This was followed in 2009 by the publication of results from another phase I/II clinical trial involving patients who also had metastatic skin cancer. mRNA encoding specific antigens was injected directly and achieved a complete decrease in cancer cells in one patient.


Finally, in 2012, the Institute of Immunology at the Friedrich Loeffler Institute in Tübingen and CureVac announced in a joint publication that mRNA vaccines built up a protective effect against influenza A viruses (true flu viruses) in mice.
This put CureVac at the forefront of mRNA vaccine development against infectious diseases.
Katalin Karikó and Drew Weissman from UPenn
Also in 2000, in October, U.S. scientists Drew Weissman and Katalin Karikó of the University of Pennsylvania (UPenn), together with other colleagues, published a paper on the successful induction of an in vitro immune response using mRNA transfected dendritic cells. The experiments were performed on cell cultures in Petri dishes, but not in vivo at that time, for example, on living mice. These became sick, their immune system reacting with inflammation to the "foreign" mRNA.
They continued to work on their technology and were able to reduce immunogenicity of both mRNAs by exchanging a base building block, which they published in an article in August 2005 and applied for a patent in 2006. Sascha Karberg, who wrote a biography of Ingmar Hoerr, summarizes the investigations as follows: "... which 'guardian' proteins recognize foreign RNA and trigger the emergency reaction of the cells, and with which tricks viruses such as influenza, which use RNA as a hereditary molecule, manage to remain undetected by them. The two scientists observed that the viruses modify their mRNA, creating a kind of cloak of invisibility by attaching chemical appendages (such as methyl groups) to the RNA building blocks A, C, G, or U. They were able to identify the viruses by means of the mRNA and the methyl groups. Following this lead, they finally made a breakthrough in 2005: when they exchanged the uridine building block of the mRNA for an artificial pseudouridine building block that does not occur in nature, the mRNA escaped the attention of the guardian proteins but was still effectively translated into protein."




Stephen Buranyi writes in his article that Karikó had been working on mRNA since 1989. Searches in PubMed.gov reveal that her first co-publication, with the term mRNA in its title, appeared in 1990. However, in that publication, as well as others, the focus was less on investigating the use of mRNA as a vaccine. Rather, it was about optimizing the method of gene transfer (for example, article from 1998) as well as instructing cells to make certain proteins (article from 1999).
Then something happened that often takes scientific research further: meeting or exchanging ideas with colleagues. Already the get together at Cambridge University in April 1960 brought Brenner and Jacob together (see part 1 of the blog series), helping to elucidate the mysterious molecule "X" as mRNA. In the late 1990s, it was a chance meeting that took researchers at UPenn further in the matter of mRNA as a drug or vaccine.
Happening in 1998 at the photocopier shared by two labs, the emerging conversation, according to Gina Kolata, was as follows. "Dr. Weissman happened by, and she {Kariko} struck up a conversation. 'I said, ‘I am an RNA scientist — I can make anything with mRNA,’' Dr. Kariko recalled. Dr. Weissman told her he wanted to make a vaccine against H.I.V. 'I said, ‘Yeah, yeah, I can do it,’' Dr. Kariko said." Weissman had been at UPenn since 1997, coming from the Laboratory for Immunoregulation at the National Institutes of Allergy and Infectious Diseases (Facility of the National Institutes of Health, NIH) where he conducted research on HIV/AIDS together with Anthony Fauci.
The final path to drug technology
The 2000s publications started a new head-to-head race on how to stabilize short-lived mRNA and how to make it less immunogenic. The goal in each case was to use mRNA as a drug technology: Cell reprogramming, gene therapies, protein replacement therapies, gene silencing, cancer immunotherapies, or virus vaccines. The following selected additional milestones were achieved (first author only):
- 2002 | First clinical U.S. trial (Duke University) with dendritic cells, ex vivo transfected with mRNA, coding for PSA (prostrate specific antigen) to treat prostrate cancer. On board Eli Galboa, founder of Merix Bioscience | A. Heiser
- 2004 | Stabilized mRNA molecules show immuno stimulating capacities | B. Scheel (CureVac)
- 2006 | Modification of antigen-encoding RNA increases stability, translationals efficacy, and T-cell stimulatory capacity of dendritic cells | S. Holtkamp (University of Mainz, co-authors Sahin & Türeci)
- 2008 | Pseudouridin in mRNA increases translation and biological stability while reducing immunogenicity | K. Karikó
- 2009 | First adoptive immunotherapy using mRNA-targeted receptors from immune system cells | S. Yoon
- 2010 | Reprogramming of stem cells using synthetical modified mRNA | L. Warren
- 2011 | HPLC purification eliminates immune activation and increases translation of nucleoside-modified mRNA | K. Karikó
- 2011 | Preclinical protein replacement study: nucleoside-modified mRNA leads to erythropoietin (EPO) formation; potential alternative to DNA-based gene therapy. | K. Karikó
- 2013 | First mRNA Konferenz in Tübingen, launched by CureVac
- 2015 | First clinical study on mRNA vaccination in prostrate cancer | H. Kübler (CureVac)
- 2017 | Publications and patent filings on mRNA-vaccines against HI, Zika, Hepatitis C and Herpes viruses
- 2017 | First personalized mRNA vaccine, tested in patients with skin cancer | U. Sahin (BioNTech)
- 2017 | Elimination of large tumors in mice using mRNA encoded bispecific antibodies | C. Stadler (BioNTech)
- 2019 | Study on mRNA based cancer immunotherapy in patients with non small cell lung cancer | M. Sebastian (CureVac)
In addition to CureVac, BioNTech from Mainz, Germany, and ModeRNA from Cambridge, USA, founded in 2008 and 2010, respectively, emerged as companies.
Part 4 of this blog series will further elaborate on these companies as well as highlight their contribution to the development of mRNA vaccines.
Sources used:
- Chiranjib Chakraborty et al., Adamas University in India, frontiers in Immunology (Jul 2021)
- Sascha Karberg, "Der Mann, der das Impfen neu erfand", Aufbau Verlag (Jun 2021)
- Stephen Buranyi, WIRED, published in "LONG READS" (Juni 2021)
- Gina Kolata, NY Times (Apr 2021)
- Ugur Sahin, Katalin Karikó and Özlem Türeci, BioNTech SE , Nature (Sep 2014); image source cover image