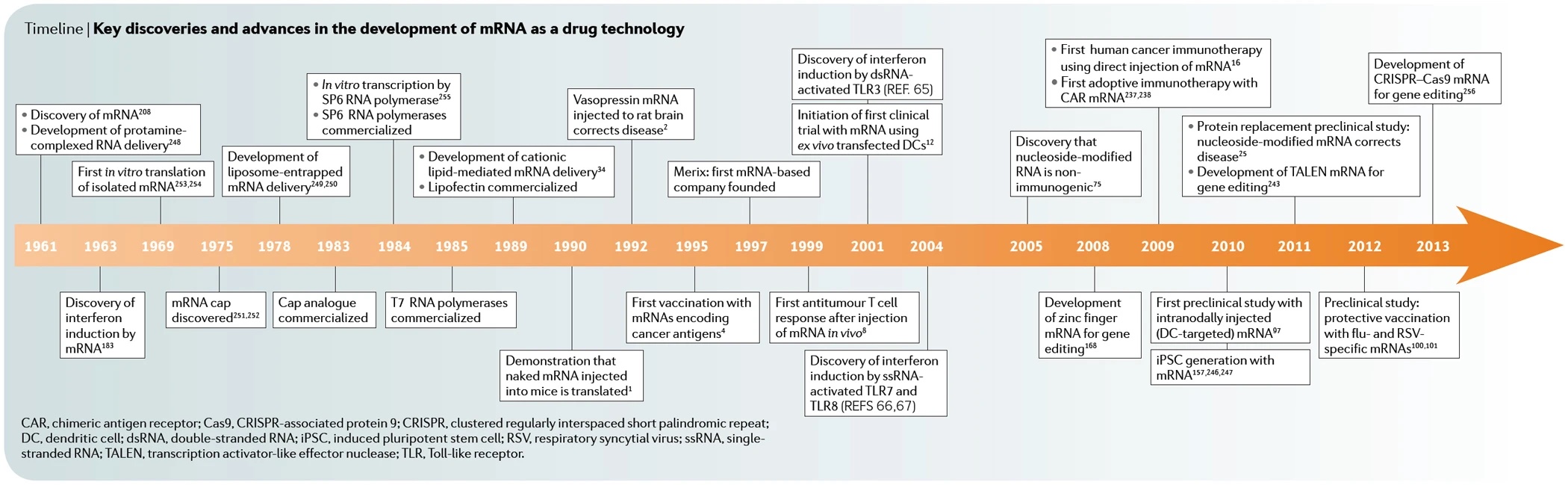
Following the approval of the world's first gene therapy for a specific form of retinitis pigmentosa (RP) in 2017, researchers now report on another gene therapy approach to treat this disease using optogenetics. Medical researchers from the U.S. and Switzerland were able to help a 58-year-old man who had been blind for decades due to the retinal disease to recognize and grasp objects again.
With the help of special glasses, the patient was able to recognize a telephone, a door or a crosswalk, as the team led by José-Alain Sahel of the University of Pittsburgh School of Medicine and Botond Roska of the University of Basel described in Nature Medicine in May 2021.
In RP, certain genes that code for light receptors in the retina are mutated. More than 70 genes are known whose non-function results in the gradual loss of vision. According to the authors, more than two million people worldwide are affected; in Germany, the number is estimated at up to 40,000. To date, there is only one approved therapy for this inherited disease. This only applies to early RP, which is triggered by a mutation in the RPE65 gene and in which the light receptor cells have not yet died.
Worldwide first RP gene therapy
In December 2017, the U.S. FDA approved the first gene therapy for a hereditary eye disease that can lead to blindness as early as age of 20. LUXTURNA (Voretigene neparvovec) was developed by the U.S. biotech company Spark Therapeutics, founded in 2013. Approval in Europe by the EMA (European Medicines Agency) followed in 2018. Novartis from Switzerland secured global marketing rights - with the exception of the U.S.

Mode of action of Luxturna
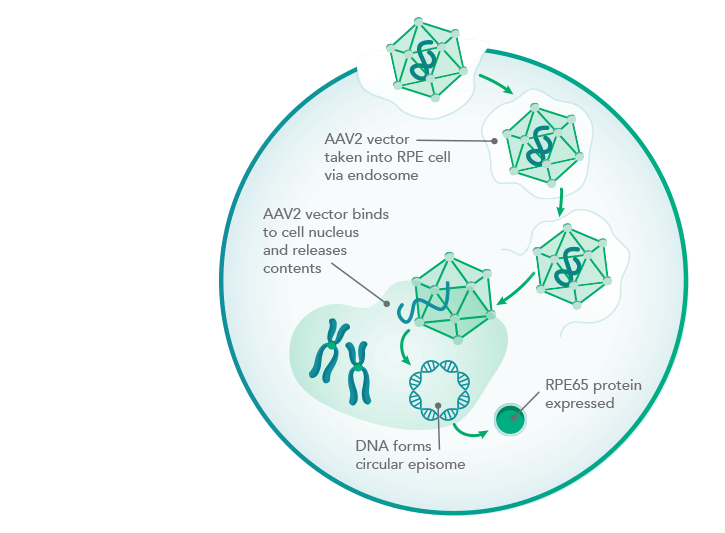
Mode of action of Luxturna
In December 2019, Roche, also based in Switzerland, paid $4.3 billion to acquire Spark, which will remain an independent subsidiary.
LUXTURNA uses as its gene delivery vehicle a virus that provides retinal cells with a functional copy from the RPE65 gene. The RPE65 protein encoded by this is a visual pigment missing in the disease. With a price tag of $850,000, the therapy is one of the most expensive on the U.S. market.
What is optogenetics and how does it treat blind people?
Optogenetics is basically about introducing genetic information into target cells that codes for light-sensitive ion channels, transporters or enzymes. Ultimately, cellular activities can be controlled with light.
The starting point was research by Peter Hegemann's team at the Humboldt University in Berlin, which studied the green alga Chlamydomonas in the 1990s and discovered so-called channel rhodopsins. These proteins act like switches in certain cells that react to light.
In 2004, Karl Deisseroth's team at Stanford University succeeded in inserting the gene of these algal proteins into the nerve cells of a mouse. They then produced the protein and became light-sensitive. This made it possible to control the mouse remotely by light impulses: it ran around in circles, stopped and started running again.
Since then, Deisseroth has been regarded as the founder of optogenetics, i.e. the control of genetically modified cells with the aid of light. The technique was named Method of the Year 2010 by Nature Methods.
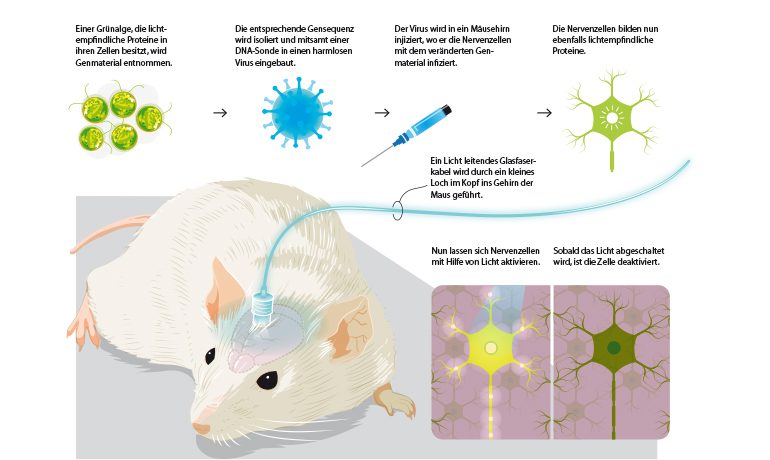
By means of a fiber optic cable inserted into the brain, nerve cells can be activated or deactivated in a mouse experiment. Image source: KlarText
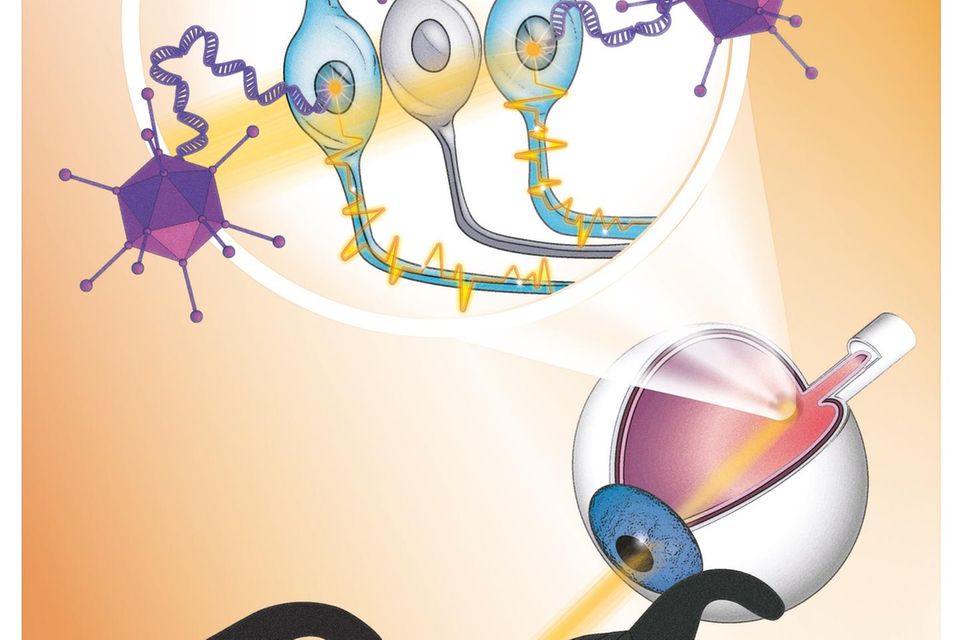
New gene therapy makes a blind person's retina sensitive to light, aided by light-stimulating goggles that use a neuromorphic camera to capture images from the visual world. Image source: GEO
For the therapy of blind patients, optogenetics offers the advantage that it can be used independently of mutations. This is because new light-sensitive molecules are introduced into the eye and no attempt is made to replace non-functioning ones by gene transfer.
In the study, the team injected a single dose of viruses into the man's more severely affected eye, which transferred the blueprint for the light-sensitive protein derived from microalgae (canal rhodopsin). This forms an ion channel in certain cells of the lower retina. This is needed to generate electrical signals from incident light, which the cells transmit to the visual center in the brain. Since daylight is not sufficient to activate the ion channel, camera glasses assist by projecting images of the surroundings through the pupil onto the retina in real time at a very high intensity.
The patient started training with the glasses four and a half months after the injection. This was the period needed for the target cells to form ion channels in the inferior retina. Then, after seven months, he reported visual impressions, but only when wearing the glasses. However, the treating physicians warn against exaggerated hopes. At present, it cannot be expected that patients will be able to recognize faces or even read after such a therapy. Nevertheless, the procedure can significantly improve the quality of life - especially since the 58-year-old was treated with only a low dose.
Peter Hegemann speaks of a "breakthrough into a new era for the treatment of retinitis pigmentosa". However, clinical use is "still a long way off and many things can be improved in the near future," emphasizes the Berlin neuro-scientist, who is one of the discoverers of canal rhodopsins.
Sources used:
- Article in Geo dated on May 25, 2021
- Article in Süddeutsche Zeitung dated on May 25, 2021
- Original article by Sahel et al., Partial recovery of visual function in a blind patient after optogenetic therapy, Nature Medicine, published online on May 24, 2021
- Article in KlarText, Controlling cells with light - and love, insert by Joachim Schüring